Polymer #4: Poly(tetrafluoroethene)
(Also known as poly(tetrafluoroethylene) or PTFE.)
Tetrafluoroethene is an example of a fluorocarbon, a compound of fluorine and carbon. In the molecule, all four hydrogen atoms of ethene have been replaced by fluorine atoms.
Fluorocarbon compounds constitute almost another world from organic and inorganic chemistry, in part because the C-F bond is so strong that it almost totally ignores attack by other molecules. In the small fluorine atoms, the highly charged fluorine nucleus exerts such tight control over the electrons in its vicinity that they take much less part in weak intermolecular bonding than do the electrons in hydrocarbons. As a result, fluorocarbons are generally more volatile than the corresponding hydrocarbons.
Fluorocarbons came into prominence after World War II, when the nuclear industries grew and supplies of fluorine became available (fluorine is used in the manufacture of uranium hexafluoride, UF6, a volatile solid used in the separation of uranium isotopes). Tetrafluoroethene is a colourless, odourless, tasteless gas; its principle destination is polymerisation to give the fluorocarbon analogue of poly(ethene).
PTFE consists of very long chains, composed of about 50000 -CF2- groups each, with very little cross-linking between them. As a result, the molecules pack together to give a dense, compact solid with a high melting point. Even when the material is molten, the chains are so closely packed that they flow past each other only very slowly. Molten PTFE is so viscous that most PTFE articles are made by heating and compressing the powder to obtain a dense, strong, homogeneous lump.
The chemical and thermal stability of PTFE can be traced to two features. One is the considerable strength of the C-C and C-F bonds, which keeps the molecules from decomposing even they are moderately heated. The second feature is the match between the sizes of the fluorine and carbon atoms, which results in the fluorine atoms forming an almost continous sheath around the chain of carbon atoms, protecting it from chemical attack. In effect, the fluorine atoms act as chemical insulation around the carbon-atom 'wire'.
Grease and oil do not form bonds with PTFE, so surfaces coated in it are 'non-stick'; that is, PTFE is an abherent coating, the opposite of an adherent substance like glue. Because fats and oils do not form bonds with the alien PTFE molecules, PTFE feels slippery to the touch. Its molecules pack together so densely that the solid does not absorb water, making it an excellent electrical insulator.

Tetrafluoroethene is often copolymerised with other fluorocarbons to produce the range of plastics widely known as Teflons (a Du Pont trade name). One Teflon is PTFE itself. Another, Teflon-FEP (fluorinated ethene-propene), is a copolymer of tetrafluoroethene and the fully fluorinated version of propene (CF3-CF=CF2). The -CF3 groups of the fluoropropene molecule are bumps on the -CF2- backbone of the copolymer and result in less close packing. As may be suspected, the solid melts at a lower temperature than does PTFE and forms a liquid with a lower viscosity. It can therefore be moulded by conventioonal (injection) techniques but retains the desirable thermal and chemical stability of PTFE.
Tetrafluoroethene is an example of a fluorocarbon, a compound of fluorine and carbon. In the molecule, all four hydrogen atoms of ethene have been replaced by fluorine atoms.
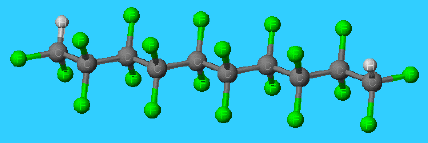
Molecular structure of PTFE

Fluorocarbon compounds constitute almost another world from organic and inorganic chemistry, in part because the C-F bond is so strong that it almost totally ignores attack by other molecules. In the small fluorine atoms, the highly charged fluorine nucleus exerts such tight control over the electrons in its vicinity that they take much less part in weak intermolecular bonding than do the electrons in hydrocarbons. As a result, fluorocarbons are generally more volatile than the corresponding hydrocarbons.
Fluorocarbons came into prominence after World War II, when the nuclear industries grew and supplies of fluorine became available (fluorine is used in the manufacture of uranium hexafluoride, UF6, a volatile solid used in the separation of uranium isotopes). Tetrafluoroethene is a colourless, odourless, tasteless gas; its principle destination is polymerisation to give the fluorocarbon analogue of poly(ethene).
PTFE consists of very long chains, composed of about 50000 -CF2- groups each, with very little cross-linking between them. As a result, the molecules pack together to give a dense, compact solid with a high melting point. Even when the material is molten, the chains are so closely packed that they flow past each other only very slowly. Molten PTFE is so viscous that most PTFE articles are made by heating and compressing the powder to obtain a dense, strong, homogeneous lump.
The chemical and thermal stability of PTFE can be traced to two features. One is the considerable strength of the C-C and C-F bonds, which keeps the molecules from decomposing even they are moderately heated. The second feature is the match between the sizes of the fluorine and carbon atoms, which results in the fluorine atoms forming an almost continous sheath around the chain of carbon atoms, protecting it from chemical attack. In effect, the fluorine atoms act as chemical insulation around the carbon-atom 'wire'.
Grease and oil do not form bonds with PTFE, so surfaces coated in it are 'non-stick'; that is, PTFE is an abherent coating, the opposite of an adherent substance like glue. Because fats and oils do not form bonds with the alien PTFE molecules, PTFE feels slippery to the touch. Its molecules pack together so densely that the solid does not absorb water, making it an excellent electrical insulator.
PTFE non-stick cookware

Tetrafluoroethene is often copolymerised with other fluorocarbons to produce the range of plastics widely known as Teflons (a Du Pont trade name). One Teflon is PTFE itself. Another, Teflon-FEP (fluorinated ethene-propene), is a copolymer of tetrafluoroethene and the fully fluorinated version of propene (CF3-CF=CF2). The -CF3 groups of the fluoropropene molecule are bumps on the -CF2- backbone of the copolymer and result in less close packing. As may be suspected, the solid melts at a lower temperature than does PTFE and forms a liquid with a lower viscosity. It can therefore be moulded by conventioonal (injection) techniques but retains the desirable thermal and chemical stability of PTFE.
0 Comments:
Post a Comment
<< Home