Polymer #3: Poly(chloroethene)
(Also known as poly(vinyl chloride) or PVC.)
Now we let the ethene molecule acquire a bit more personality by replacing a hydrogen atom with a chlorine atom. At first sight, the new personality is unattractive, for chloroethene (or vinyl chloride) is a carcinogenic gas. Nevertheless, it is manufactured (from ethene) in huge amounts each year, since it can be polymerised to form PVC, one of the most useful and adaptable of all plastics.
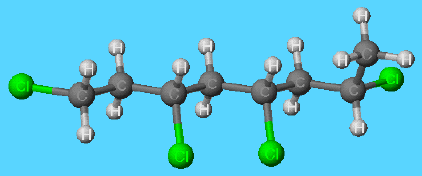
Because of the big chlorine atoms present on the chains, PVC molecules do not pack together so well that they form a rigid solid. However, to increase the flexibility of the solid, commercially produced PVC molecules do contains large organic molecules (including esters of alcohols containing about ten carbon atoms) that act as plasticisers that lubricate the PVC molecules and allow them to move easily past each other when the solid is bent. These lubricants are not bound chemically to the polymer chains and slowly migrate to the surface. There they are lost and, as a result, the plastic becomes brittle and stiff. Since some bacteria enjoy a good meal of hydrocarbon chains and will eat parts of the plasticiser molecules, biocides are somtimes incorporated into PVC.
PVC is produced in such huge amounts because it is so versatile. It can be mixed with a very wide range of additives chosen to tailor its properties to many different applications. When it is properly protected by its additives, it is also chemically resistant to attack and degradation.
Now we let the ethene molecule acquire a bit more personality by replacing a hydrogen atom with a chlorine atom. At first sight, the new personality is unattractive, for chloroethene (or vinyl chloride) is a carcinogenic gas. Nevertheless, it is manufactured (from ethene) in huge amounts each year, since it can be polymerised to form PVC, one of the most useful and adaptable of all plastics.
Molecular structure of PVC

Because of the big chlorine atoms present on the chains, PVC molecules do not pack together so well that they form a rigid solid. However, to increase the flexibility of the solid, commercially produced PVC molecules do contains large organic molecules (including esters of alcohols containing about ten carbon atoms) that act as plasticisers that lubricate the PVC molecules and allow them to move easily past each other when the solid is bent. These lubricants are not bound chemically to the polymer chains and slowly migrate to the surface. There they are lost and, as a result, the plastic becomes brittle and stiff. Since some bacteria enjoy a good meal of hydrocarbon chains and will eat parts of the plasticiser molecules, biocides are somtimes incorporated into PVC.
PVC pipes

PVC is produced in such huge amounts because it is so versatile. It can be mixed with a very wide range of additives chosen to tailor its properties to many different applications. When it is properly protected by its additives, it is also chemically resistant to attack and degradation.
0 Comments:
Post a Comment
<< Home