Polymer #2: Poly(propene)
Many polymers are variations on the theme of poly(ethene), differing only by having one or more of the hydrogen atoms in the monomer molecule replaced by other atoms or groups. The propene molecule is an ethene molecule in which a methyl (-CH3) group has replaced one hydrogen atom.
Molecular structure of poly(propene)
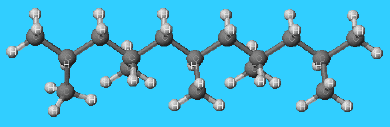
It is obtained together with ethene when hydrocarbons are cracked, and it can be polymerised to form long molecules with a poly(ethene)-like backbone and methyl groups on alternate carbon atoms. Special catalysts are used in order to ensure that there is little chain branching and that the methyl groups all point in the same direction. Such an orderly polymer is said to be isotactic, and most of the poly(propene) available commercially is of this kind.
Poly(propene) ropes

The molecules in an isotactic polymer can lie close together, giving an extremely orderly solid. Because of its orderliness (or crystallinity), poly(propene) is stiff, hard, and resistant to abrasion and has a high enough melting point for objects made from it to be sterilised. However, because the methyl groups are liable to undergo oxidation, poly(propene) articles usually have antioxidants incorporated into them to divert attack by oxygen. Because poly(propene) is a kind of frozen oil, unlike nylon it does not absorb water and is resistant to discolouring; these properties make it suitable for outdoor carpeting.
Poly(propene) carpet

More polymers: poly(ethene), poly(chloroethene), poly(tetrafluoroethene).
Adapted from "Atkins' Molecules" by Peter Atkins
4 Comments:
Hi, will you also write about PVC (poly-vinilchloride)?? :D
Yep, will post PVC soon.
btw, PVC = poly(chloroethene)
Oh, i see, that's probably just about language problem. Here, PVC = poly(vinilchloride).
- CHCl - CH2 - << this is the structure of one vinilchloride (or chloroethene), just the same actually.... :) Only about naming... :D
Post a Comment
<< Home